General Disclaimer
Intended Use
- Shield is a qualitative in vitro diagnostic test intended to detect colorectal neoplasia by identifying genomic and epigenomic alterations in cell-free DNA.
- Shield is intended for colorectal cancer screening in individuals at average risk for the disease, age 45 years or older, and is not intended to be the sole basis for a diagnosis of colorectal cancer.
Important Safety Information
- Shield is for prescription use only. This test should not be used if you have:
- A personal history of colorectal cancer (CRC)
- A family history of CRC, defined as having one or more first-degree relative (parent, sibling, or child) diagnosed with CRC at any age
- A known hereditary / germline risk of CRC (for example, Lynch syndrome or Hereditary Non-Polyposis CRC, or Familial Adenomatous Polyposis)
- A known diagnosis of inflammatory bowel disease
- False positive and false negative results may occur.
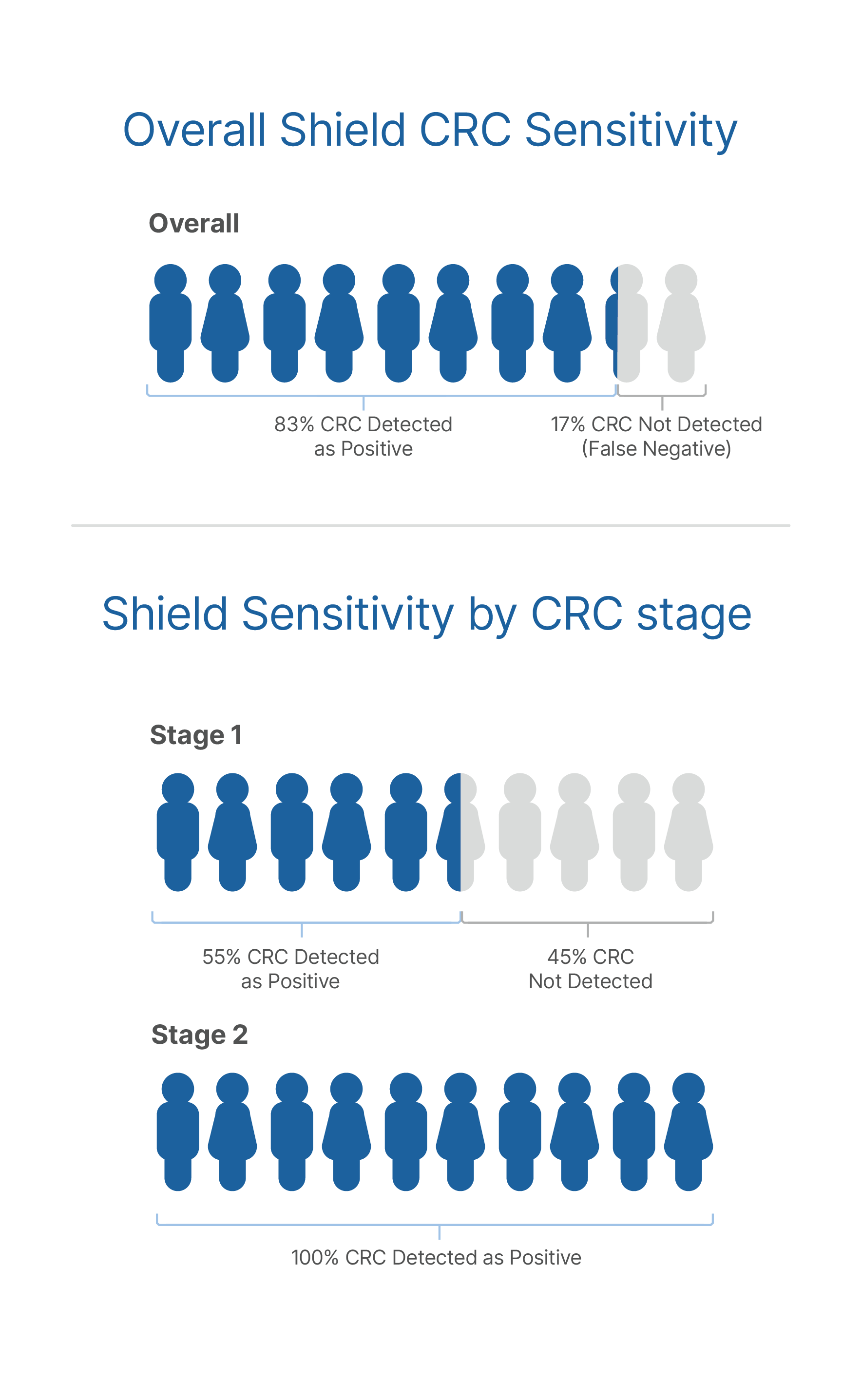
- An Abnormal Signal Detected suggests the presence of colorectal cancer or advanced adenoma. Patients with an Abnormal Signal Detected result should be referred for colonoscopy evaluation.
- Shield has limited ability to detect advanced adenomas.
- A normal Shield result does not rule out colorectal cancer and patients should continue participating in guideline-recommended screening programs.
- Shield should be considered alongside other CRC screening modalities, like colonoscopy, and is not a replacement for diagnostic or surveillance colonoscopy of high-risk individuals.
Please click here for full patient information