CANCER SCREENING
Thanks to Shield™, you can help improve colorectal cancer screening adherence
Want to learn more about the Shield blood test? Complete the form below to stay up to date
*Required fields
Thank you!
We’ve received your information. A Shield team member will be reaching out to you in the next 24-48 business hours to address your questions.
Please check your inbox for an email with helpful information in the meantime.
We’ve received your information, and your first email is on its way to help address any questions. Be sure to check your inbox for regular updates from Shield.
Important Product Information
Intended Use
The Shield™ test is a qualitative, in vitro diagnostic test intended to detect colorectal cancer derived alterations in cell-free DNA from blood collected in the Guardant Shield Blood Collection Kit.
Shield is intended for colorectal cancer screening in individuals at average risk for the disease, age 45 years or older. Patients with a positive result should be followed by colonoscopy. Shield is not a replacement for diagnostic colonoscopy or for surveillance colonoscopy in high-risk individuals. This test is performed at Guardant Health, Inc.
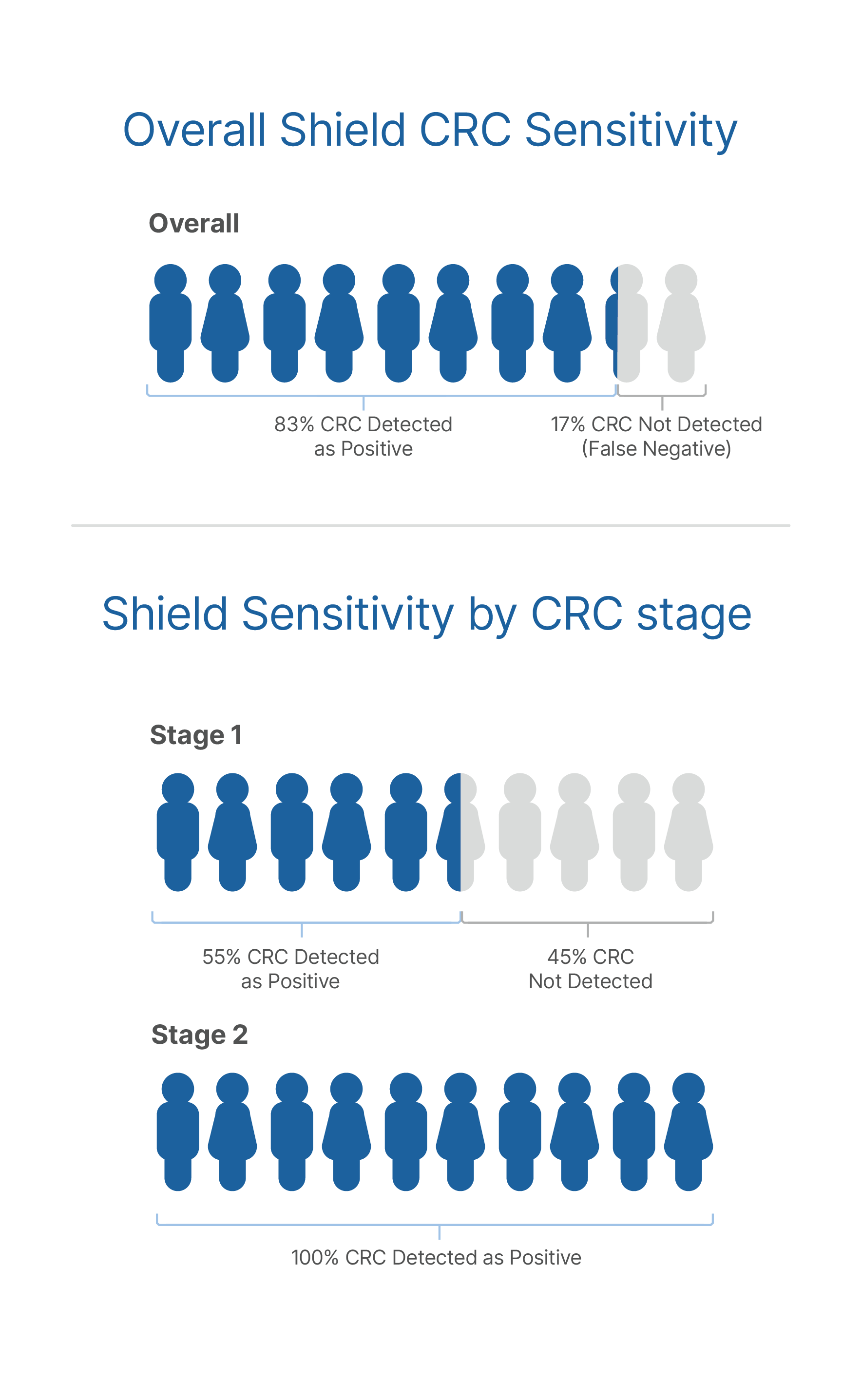
Precaution: Based on data from clinical studies, Shield has limited detection (55%-65%) of Stage I colorectal cancer and does not detect 87% of precancerous lesions. One out of 10 patients with a negative Shield result may have a precancer that would have been detected by a screening colonoscopy. Shield demonstrated high detection of Stages II, III, and IV colorectal cancer.
Limitations and Other Important Information: The Shield test is not intended for patients that have personal history of colorectal cancer, adenomas, or other related cancers; or those who had a positive result on another colorectal cancer screening method within the last six months, have been diagnosed with a condition associated with high risk for colorectal cancer such as Inflammatory Bowel Disease (IBD), chronic ulcerative colitis (CUC), Crohn’s disease; or who have a family history of colorectal cancer, or certain hereditary syndromes. Providers should discuss the most appropriate screening test to use with patients depending on their medical history and individual circumstances. Shield may produce a false negative or false positive result. A false negative result may occur when the Shield test does not detect a colorectal tumor signal while a colonoscopy identifies a colorectal cancer. A negative result does not guarantee the absence of colorectal cancer or advanced adenoma, and patients should continue participating in colorectal cancer screening programs at the appropriate guideline-recommended intervals. A false positive result may occur when the Shield test generates a positive result while a colonoscopy will not find colorectal cancer or advanced adenoma.
Please read our Provider Brochure for complete product information about the Shield test including full safety information.