CANCER SCREENING
Make screening easy with Shield™
Improve adherence with the ability to screen right in your office with Shield, an accurate, FDA-approved blood test for CRC screening1,2
Screen with ShieldShield can help overcome screening challenges1
The only FDA-approved blood test available for CRC screening with Medicare coverage
Convenient
Can be completed in your office at any patient visit
Quick
You will receive test results in ~2 weeks
Simple
As easy as doing a complete blood count
Offer Shield as a CRC
screening option
Help patients choose a screening method that works for them with shared decision making
2.4x
increase in screening rates when Shield was offered1
Alongside colonoscopy and FIT. Based on a study of over 1000 patients, age 45-75, at a large, integrated health system. There was a statistically significant difference in screening completion rate between the "usual care" group (FIT and colonoscopy), and the "intervention" group (usual care + Shield). Data based on Laboratory Developed Test (LDT) usage of Shield, which has not been cleared or approved by the FDA
Order ShieldWith the Shield blood test,
you finally get accuracy and adherence1,2
The ECLIPSE study, published in the New England Journal of Medicine, included2:
- More than 20,000 average-risk individuals, ages 45-84*†
- A diverse population from over 200 clinical trial sites and 34 states that reflects US demographics
Blood-based cell-free DNA (bb-cfDNA) testing: the only first-line FDA-approved CRC screening blood test recommended by the NCCN Guidelines®3
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN=National Comprehensive Cancer Network.
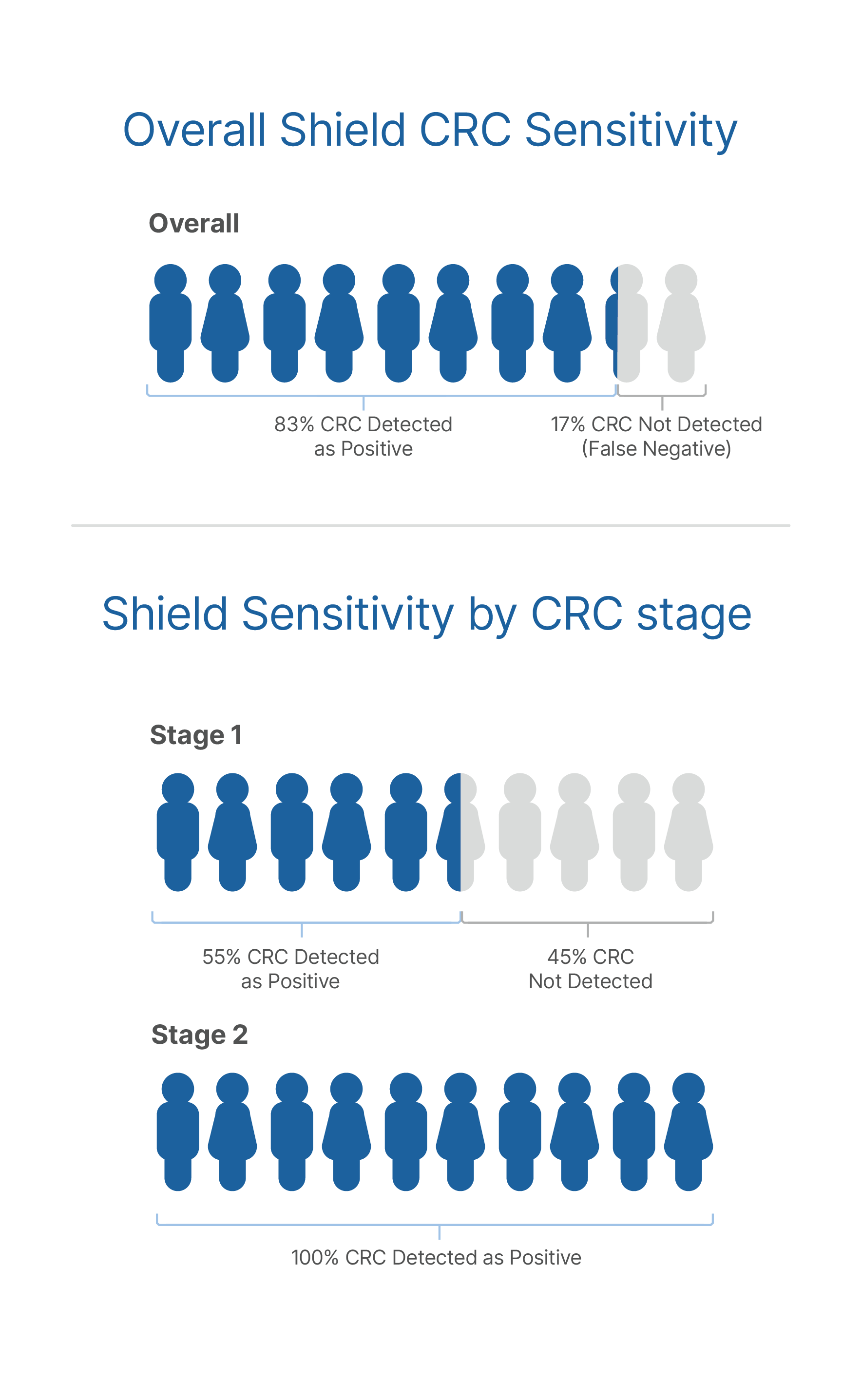
ECLIPSE demonstrated Shield's
ability to accurately detect CRC2
overall CRC sensitivity
specificity

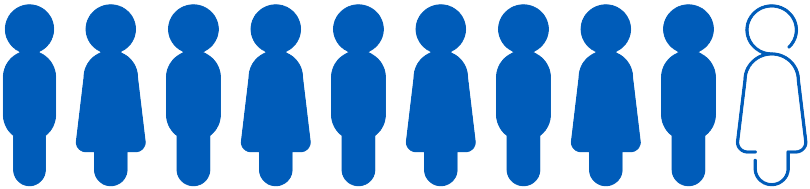
Of the first 20,000 patients offered the Shield LDT,
9 out of 10 patients
completed screening with Shield4‡
*Patients had no prior diagnosis of CRC, inflammatory bowel disease, or hereditary predisposition to CRC (eg, Lynch syndrome)
†The clinical validation cohort included over 10,000 patients. 7861 patients were included in the final clinical validation cohort
‡Internal data on file, March 2024. Data from real-world clinical settings. Data based on LDT usage of Shield, which has not been cleared or approved by the FDA
Shield is a solution for low CRC screening adherence1,2
The barriers inherent to traditional modalities prevent people from getting screened, which can lead to late or missed diagnoses5,6
*Internal data on file, March 2024. Data based on LDT usage of Shield, which has not been cleared or approved by the FDA

“With the Shield test, I’m 100% confident that the patient has gotten the test done... because we draw blood in our office”
Family Practice
Physician

Ready to offer Shield to your patients?
Start ordering today—or connect with our team to set up your practice.

- References:
- Coronado GD, Jenkins CL, Shuster E, et al. Blood-based colorectal cancer screening in an integrated health system: a randomised trial of patient adherence. Gut. 2024;73(4):622-628. doi:10.1136/gutjnl-2023-330980
- Chung DC, Gray DM II, Singh H, et al. A cell-free DNA blood-based test for colorectal cancer screening. N Engl J Med. 2024;390(11):973-983. doi:10.1056/NEJMoa2304714
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology® (NCCN Guidelines®) for Colorectal Cancer Screening. V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed August 6, 2025. To view the most recent and complete version of the guideline, go to NCCN.org.
- Data on file. Guardant Health, Inc.
- Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: A mixed-methods analysis. Am J Prev Med. 2009:38(5):508-516. doi:10.1016/j.amepre.2010.01.021
- Meester RGS, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann Epidemiol. 2015;25(3):208-213.e1. doi: 10.1016/j.annepidem.2014.11.011
- National Cancer Institute. NCI Dictionary of Cancer Terms: negative predictive value. Accessed May 21, 2024. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/negative-predictive-value
- Shield™ Provider Brochure. Redwood City, CA: Guardant Health, 2024.