CANCER SCREENING
Shield overview & how it works
Frequently asked questions
Shield is a blood test that screens for colon cancer. It starts with a simple blood draw, and can easily be part of your routine blood testing that your healthcare provider orders
- Shield is intended for patients who are 45 years of age and older, show no symptoms, and are at average risk for colon cancer
- Patients with a positive result should be followed by colonoscopy
- Discuss colorectal cancer screening options with your healthcare provider to determine the best test for you
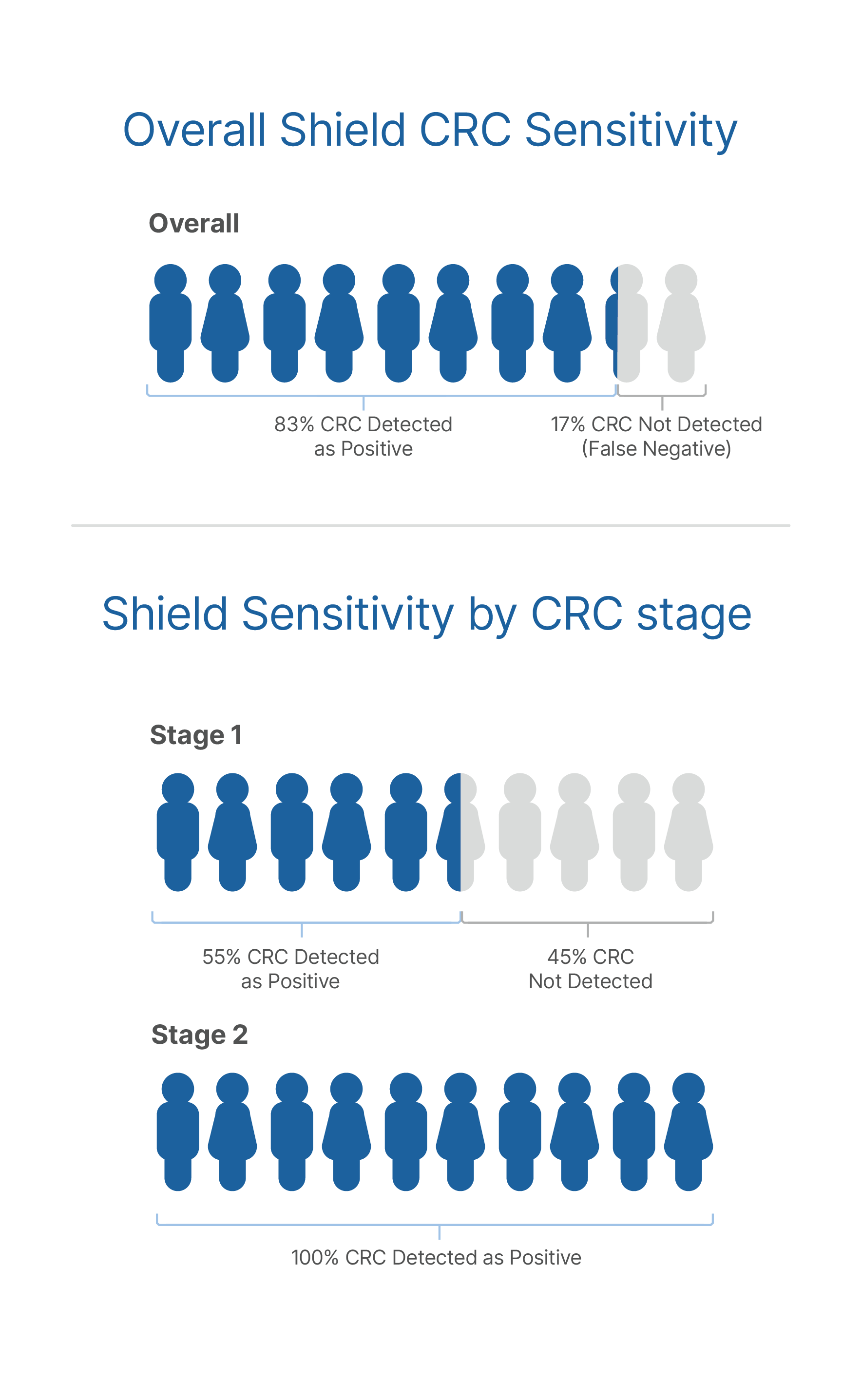
- As cancerous tumors grow, they release DNA into the bloodstream. Shield looks for this cancerous DNA to identify signals that are associated with colon cancer
- If these signals are present, the Shield test will come back with a positive result and flag a patient as needing follow-up with a colonoscopy to determine if colon cancer is present
- The Shield test is FDA-approved. FDA stands for US Food & Drug Administration. The FDA protects public health by ensuring the safety and efficacy of drugs, biologics, and medical devices, the latter of which includes in vitro diagnostic tests. For Shield to be FDA-approved, this means that the FDA has determined that there is sufficient valid scientific evidence to provide reasonable assurance that Shield is safe and effective for its intended use
Search again
Sign up for
Shield updates
Pleasant updates delivered to your inbox