CANCER SCREENING
Why screen for colon cancer with Shield™?

How Shield works:
The Shield test detects signals for colon cancer from DNA shed into the blood1,2
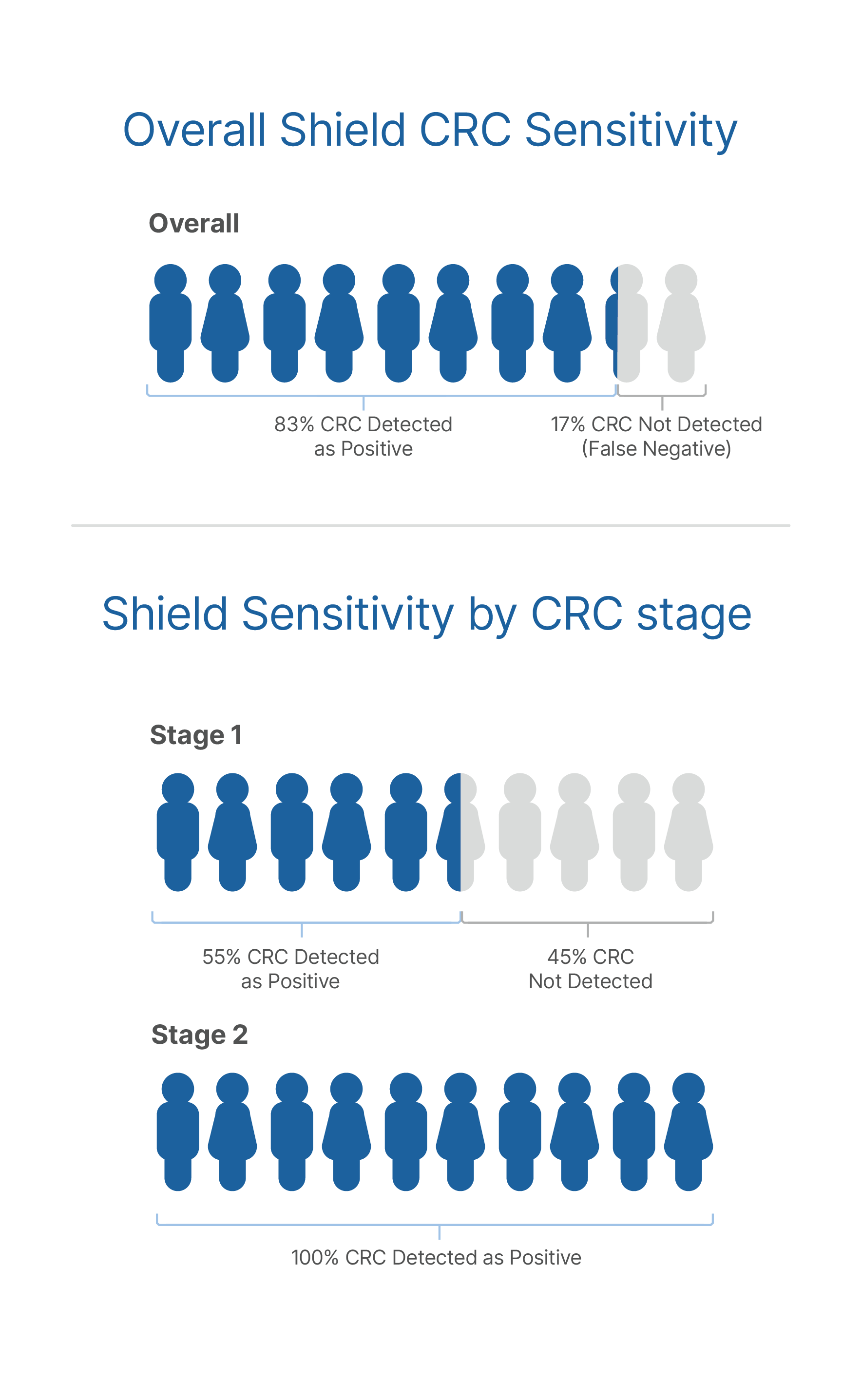
The performance of Shield has been established in the ECLIPSE clinical study of average-risk adults aged 45-84 using colonoscopy as the reference method, with over 10,000 patients: sensitivity is 83% for the detection of CRC and specificity is 90%

As cancerous tumors grow...
cancerous DNA is released into the bloodstream.
Shield looks for signals that are associated with colorectal cancer.
Patients with a "positive" result should be followed by colonoscopy. A ”negative" result indicates a signal associated with colorectal cancer was not detected.
Understanding your results
You and your healthcare provider will receive your test results in approximately two weeks
This is what your results mean*:
Negative: Signals associated with colon cancer tumors were not found2
Positive: Raises concern for the presence of a tumor in the colon or rectum. A colonoscopy is necessary to determine the presence of colorectal cancer2
Shield has limited ability to detect advanced adenomas, also known as advanced precancerous polyps
*Shield may produce a false negative or false positive result
Don’t wait. Get screened today.
Ask your doctor about the Shield blood test for colon cancer screening—from the company trusted in blood tests for cancer for over a decade.
- References:
- Chung DC, Gray DM II, Singh H, et al. A cell-free DNA blood-based test for colorectal cancer screening. N Engl J Med. 2024;390(11):973-983.doi:10.1056/NAEJMoa2304714
- Shield™ Provider Brochure. Redwood City, CA: Guardant Health, 2024